ENCINITAS, CALIFORNIA -- August 22, 2023 -- Kiora Pharmaceuticals, Inc. (NASDAQ: KPRX), a clinical-stage biotechnology company developing novel therapies for the treatment of ophthalmic diseases, today announced it has partnered with B2i Digital, Inc. to execute a comprehensive digital investor engagement and awareness program. B2i Digital will leverage its investor platform and digital marketing expertise to showcase Kiora's novel therapies for retinal diseases to retail and institutional investors. These digital efforts will be combined with traditional corporate access activities such as investor conferences, fireside chats, and Non-Deal Roadshows (NDRs).
"As we near a pivotal juncture in our clinical development pipeline, expanding access to the capital markets through innovative digital strategies is imperative," said Brian Strem, Ph.D., President & Chief Executive Officer of Kiora. "B2i Digital's leading-edge technologies will allow us to keep investors and strategic partners apprised of our progress and value proposition in a dynamic, timely manner."
"Kiora's novel therapies targeting orphan ophthalmic diseases piqued our interest from the outset," said David Shapiro, CEO of B2i Digital. "Once we examined their promising pipeline assets like KIO-301 and appreciated the potential to transform treatment for patients with retinal disease, we knew our digital investor engagement platform could significantly expand Kiora's investor base. We welcome them into our exclusive community of featured companies, telling their story to the thousands of daily b2idigital.com visitors, our 410,000+ social media community of sophisticated investors, and making individual introductions to key influencers in the capital markets."
"Our data-driven digital approach targets the most relevant investors,” added David Shapiro. “For Kiora, our platforms will generate broad awareness of their novel treatments for retinal diseases and showcase their immense potential in ophthalmology using our full suite of digital tools and platforms."
About B2i Digital, Inc.
B2i Digital, Inc. leverages the latest digital marketing technologies to tell a company's story to retail investors, institutional investors, and research analysts. B2i Digital creates robust profiles for companies on its platform, b2idigital.com, and launches targeted digital marketing campaigns to bring the most relevant investors to each company based on its sector, stage in its capital markets evolution, and overall company story. The company was founded in 2021 by David Shapiro, previously the Chief Marketing Officer for Maxim Group LLC and its investor awareness platform, M-Vest.com.
B2i Digital on Social Media:
About Kiora Pharmaceuticals, Inc.
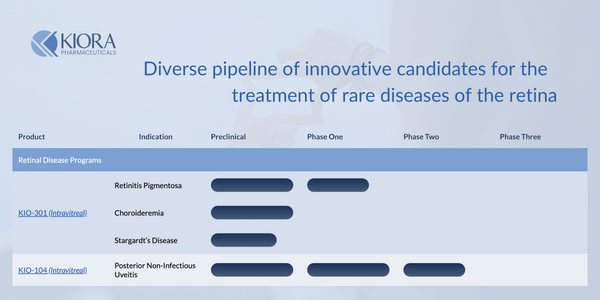
Kiora Pharmaceuticals (NASDAQ: KPRX) is a clinical-stage biotechnology company developing innovative therapies to treat rare retinal diseases like Retinitis Pigmentosa, Choroideremia, and Stargardt's Disease. The company's lead asset, KIO-301, is a first-in-class molecular photoswitch designed to restore vision in patients with inherited and age-related retinal degeneration. KIO-301 works by converting light signals in the eye into electrical signals that are transmitted to the brain. Kiora also plans to develop KIO-104 to treat non-infectious uveitis, an inflammatory eye condition. KIO-104 is a next-generation, immuno-modulatory small molecule inhibitor that blocks inflammation pathways underlying uveitis. With its promising pipeline, Kiora aims to transform the treatment landscape for patients with vision-threatening retinal diseases.
In addition to news releases and SEC filings, we expect to post information on our website, www.kiorapharma.com, and social media accounts that could be relevant to investors. We encourage investors to follow us on Twitter and LinkedIn and visit our website and/or subscribe to email alerts.
Forward-Looking Statements
Some of the statements in this press release are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These "forward-looking" statements include statements relating to, among other things, the development and commercialization efforts and other regulatory or marketing approval efforts pertaining to Kiora's development-stage products, including KIO-301 and KIO-104, as well as the success thereof, with such approvals or success, may not be obtained or achieved on a timely basis or at all, the potential ability of KIO-301 to restore vision in patients with RP, the expecting timing of enrollment, dosing and topline results for the ABACUS study, the ability to develop KIO-301 for Choroideremia and Stargardt's Disease and KIO-104 for posterior non-infectious uveitis, the ability to utilize strategic relationships to develop certain product candidates, Kiora's ability to draw on its equity line of credit, and Kiora's ability to achieve the specific milestones described herein. These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this press release, including, among other things, the ability to conduct clinical trials on a timely basis, the ability to obtain any required regulatory approvals, market, and other conditions and certain risk factors described under the heading "Risk Factors" contained in Kiora's Annual Report on Form 10-K filed with the SEC on March 23, 2023, or described in Kiora's other public filings. Kiora's results may also be affected by factors of which Kiora is not currently aware. The forward-looking statements in this press release speak only as of the date of this press release. Kiora expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions, or circumstances on which any such statement is based, except as required by law.